Aluminum Sulfate as Pulsing Preservative for Export-oriented Rose Flowers under
Bahir Dar Conditions, Northwestern Ethiopia
Muluken Yayeh1, Melkamu Alemayehu2*
and Getachew Alemayehu2
1 Faculty of Agriculture and Environmental Sciences,
Debre Tabor University, Debere Tabor, Ethiopia
2 College of Agriculture and Environmental Sciences, Bahir Dar University,
Bahir Dar, Ethiopia
Corresponding author: melkalem65@gmail.com
Received: November 8, 2017 |
Accepted: December 21, 2017 |
Abstract: Rose is
one of the most important cut flowers in Ethiopia produced mostly for export
markets. Maintaining the postharvest life is the most challenging issue for
most of the floricultural enterprises in the country. Two sets of experiments
were therefore conducted in Tana Flora PLC farm, one of the biggest cut rose
producers in Bahir Dar, Northwestern Ethiopia with the objectives of identifying
the appropriate concentration of aluminum sulfate as pulsing preservative to
maintain the vase life of export oriented rose flowers. In the first set, aluminum
sulfate alone and in combination with calcium hypochlorite at different concentrations
including the experience of Tana Flora PLC, sucrose and distilled water as control
were tested for their influence on physiological status of `Maracuja` rose flowers.
In the second set, the best performed pulsing preservative (aluminum sulfate
alone) was tested at four concentrations (0ppm, 125ppm, 250ppm and 375ppm) to
identify the optimum concentration. After pulsing for about 30 hours, six cut
flowers were put in 250 ml glass flask containing distilled water. The flasks
with flowers were arranged in Complete Randomized Design with three replications
and kept in vase life testing room of Tana Flora PLC. According to the results
obtained, aluminum sulfate at the concentration of 250ppm was the best in prolonging
the vase life, in producing bigger flower head and maintaining the freshness
of flowers. The reduction in percent fresh weight during the vase life of flowers
was minimal in 250ppm aluminum sulfate pulsed flowers. The prolonged vase life
of aluminum pulsed flowers is due to better water uptake and thus stabilized
water balance in the flowers as observed in this study. Thus, it is advised
to incorporate aluminum sulfate in flower holding solution at the concentration
of 250ppm to maintain the freshness and vase life of export oriented rose flowers
and those for local market in the study area.
Keywords: cut rose, fresh weight, transpiration
rate, water balance, water uptake
1. Introduction
Rose (Rosa hybrida L.) belongs to the family Rosaceae under which more than
150 species and 1400 cultivars are consisted (Elgimabi, 2011). Rose is one of
the most popular cut flowers and has been used as garden plant since the dawn
of civilization. Rose enjoys superiority over all other flowers being extensively
used for decorative purposes and is prized for its delicate nature, beauty,
charm and aroma. Rose plants produce an exquisite floral display consisting
of many vibrant colors, shapes, sizes and perfumes (Synge, 1971; Zlesak, 2006).
Throughout the history of civilization, no other flower has been so immortalized
and integrated into daily life as the rose. It plays a unique role in various
occasions such as Mother`s Day, St Valentine’s Day, birth and even death
(ProFlower, 2012). Thus, rose is regarded as the queen of flower (Synge, 1971).
The floriculture sector in Ethiopia is flourishing from year to year. The number
of flower exporting farms as well as the types of flowers exported is in increasing
trend (Van der Maden et al., 2011). However, roses accounted more than 80% of
the cut flower production and the floriculture cultivation area in the country.
Other floricultural crops such as chrysanthemums, poinsettia and geranium, and
bouquet fillers primarily hypericum, carnation, gypsophila, allium and carthamus
are also produced in Ethiopia (EHPEA, 2008). Ethiopia is one of the top five
flower supplies in European market including Kenya, Ecuador, Columbia and Israel
and holds an impressive second place among Dutch auction suppliers. The share
of Ethiopian flowers in European exports doubled from 6% in 2005 to 12% in 2010
(Van der Maden et al., 2011). Many varieties of the so called Hybrid Tea, Intermediate
and Sweetheart roses are now produced in modern greenhouses which are mostly
concentrated around Addis Ababa, the capital city of Ethiopia (Van der Maden
et al., 2011). Recently however, floricultural enterprises are also established
and developed in other parts of the country like Bahir Dar and Hawassa.
A very important challenge of any floricultural enterprise is maintaining the
harvest quality of flowers as long as possible. As flowers are harvested, they
are literally cut off from their source of life. As living organism however,
they respire and transpire after harvesting. As a result, water will be lost
and nutrients will be broken down that in turn accelerate the aging process
and reduce the vase life of cut flowers including roses (Van der Maden et al.,
2011). The main reason for senescence of cut flowers is wilting due to which
the floral axis bent just below the flower head which stops the water supply
to the flowers (Van Doorn and De-Witte, 1997). The reduction of water supply
is mostly attributed by physiological occultation by plant itself, air embolism
or microorganisms which plug the stem xylem vessels of the flowers (Van Doorn
and De-Witte, 1997; Loubaud and Van Doorn, 2004; Särkkä, 2005; Elgimabi,
2011).
As cut roses are harvested at bud stage, they require nutrients to open. Ichimura
et al. (2003) in their experiments were able to improve the bud opening and
extend the vase life by using sucrose as source of nutrients for cut roses.
An experiment done by Lutz and Hardenburg (1968) revealed that the cut flower
should be in a healthy condition and should be free from any damages to avoid
entry point for decaying microorganisms.
To prolong the postharvest life of cut flowers, various preservative solutions
have been recommended by researchers. Such preservatives delay senescence and
extend the vase life of cut flowers. Moreover, they prevent ethylene synthesis
and pathogen development which shorten the vase life of flowers including roses
(Halevy and Mayak, 1981; Gerailoo and Ghasemnezhad, 2011).
According to Ichimura et al. (2006), aluminum sulfate (Al2(SO4)3) has been
recommended to prolong the vase life of several cut flowers and is used as an
antimicrobial compound in commercial preservative solutions. The compound acidifies
vase solution, diminishes bacterial proliferation and enhances water uptake
(Liao et al., 2000; Hassanpour et al., 2004; Tsegaw et al., 2011) and can be
used alone or in combination of sucrose (Hussen and Yassin, 2013). According
to Seyf et al. (2012), aluminum sulfate concentrations ranging from 150 to 300
mg l-l of solution have a positive effect on the vase life of cut roses. 8-hydroxyquinoline
sulfate (8-HQS) is also the other important chemicals used in flower industry
to reduce the occurrence of decaying microorganisms in vase solutions of cut
flowers (De Stigter, 1981; Nowak and Rudnicki, 1990). Silver thiosulfate (STS)
is also known to suppress autocatalytic ethylene production by inhibition of
ethylene action (Liao et al., 2000; Butt, 2003; Da Silva, 2003; Subhashini et
al., 2011).
Although increased trend both in production and foreign exchange earnings (EHPEA
and EHDA, 2011; MoTI, 2014), no researches have been conducted in identifying
suitable preservative solution to reduce postharvest losses on floricultural
crops in the country. On the other hand, since roses are mainly produced for
export market, it has been experienced a very high postharvest losses which
impact the foreign exchange earnings of the country negatively. Therefore, the
aim of this study was mainly to evaluate pulsing preservative solutions on vase
life of rose and to identify and advise the best performed preservative for
reduction of postharvest losses of export oriented rose flowers.
2. Materials and Methods
2.1. Description of the study area
The experiments were conducted in December 2015 in vase life experimental room
of Tana Flower PLC Bahir Dar, Ethiopia, which is one of the biggest producers
and exporters of cut roses in the country. The site is located at 11.710 N latitude
and 37.30 0 E longitude. The altitude of the site is about 1850 m above sea
level and the average annual rainfall and relative humidity are about 1250 mm
and 65%, respectively. The minimum and maximum temperatures of the study site
during the experimental period were about 10.5°C and 27°C, respectively.
2.2. Experimental materials and preservative solutions
The study was conducted in two sets of experiments. In the first set, the effects
of five pulsing preservative solutions including distilled water as control
were evaluated on vase life of rose variety `Maracuja`; which is a dominant
rose variety produced in Tana Flora PLC. Pulsing preservative used by Tana Flora
PLC for cut roses was also included in the experiment which is represented by
treatment four (T4) of this experiment (Table 1). In the second set of the experiment,
the best performed pulsing preservative (aluminum sulfate) was tested in four
concentrations (0ppm 125pmm, 250ppm, 375ppm) to identify the optimum and area
specific concentration of aluminum sulfate which prolog the vase life of rose
flowers.
Table 1. Pulsing preservative solutions used in the first
set of the experiment
Treatment |
Pulsing preservatives & their concentrations
|
| T1 |
Distilled water as control |
| T2 |
Al2(SO4)3 (250ppm)
|
| T3 |
Al2(SO4)3 (250ppm)b
+ Ca(ClO)2 (66.7ppm)c |
| T4a |
Al2(SO4)3 (666.7ppm)
+ Ca(ClO)2 (66.7ppm) |
| T5 |
Sucrose (20 g l-1) |
a Concentration used by Tana Flora PLC; b Aluminum sulfate;
c Calcium Hypochlorite
2.3. Handling of experimental materials and pulsing procedures
Matured and uniform sized rose buds with enclosed sepals having about 60 cm
stem length were harvested early in the morning, trimmed to 10 cm under water
to avoid water embolisms. All leaves on the lower section of the flower stems
were removed and put immediately in uniform shaped and sized flower buckets
containing the pulsing preservative solutions. The amount of preservative solutions
in the flower buckets was determined in such a way that about 15 cm of the rose
stem cuttings were covered with pulsing solutions.
For the purpose of field heat removal, flower buckets containing the pulsing
preservatives and rose stem cuttings were placed in pre-cooling room having
a temperature of about 8-10 °C for about 6 hours. After field heat removal,
the old preservative solutions were replaced by the new once having the same
concentration and amount. Flowers were then transferred into cold room with
about 2 °C room temperatures for about 24 hours for final cooling.
After cold room, the pulsing preservatives in flower buckets were replaced
with distilled water and six rose flowers were put into 250 ml experimental
flasks containing distilled water and transferred into vase life experimental
room of Tana Flora PLC with temperature ranging from 22-25°C and relative
humidity of 65-70%. The pulsing procedures followed in this study resemble the
practices of Tana Flora PLC for export rose flowers. The flasks containing six
rose flowers each were arranged in complete randomized design (CRD) with three
replications on working table. At the time of transfer to the experimental flask
about 2 cm long stem was cut off at the bottom to improve transport of water
through the flower stem.
2.4. Determination of physiological status of flowers and analysis
Fresh weight of flower stems (g/plant): The flask
was weighed with flask + solution + flowers and weight of flask and solution
was subtracted the difference in the weight signifies fresh weight of flowers.
This process was repeated everyday and weight per flower stem was computed.
Water uptake (g/flower): For determining water uptake,
flasks were weighed with the solution without flowers and the consecutive difference
in weight signifies the water uptake.
Transpiration loss (g/flower): flowers were weighted
daily along with solution and flowers, the consecutive difference in weights
represent the (existence of) transpiration loss.
Water balance: Water balance was calculated by subtracting
the total transpiration loss from water uptake.
Flower head diameter (cm): The diameters of four
randomly selected flower buds at full bloom were measured at the center using
caliper and the mean values were used for analysis.
Vase life (days): Vase life of cut flowers was determined
on the percentage of wilting. When the neck of 50% of the flowers in the flask
bent over, the vase life was terminated as described by Lama et al. (2013).
At this point discoloration and loss of petals were started (Halevy and Mayak,
1981; Liao et al., 2000). The number of days starting from harvesting until
this stage was counted and used for evaluation.
Collected data were subjected to analysis of variance (ANOVA) using SAS- computer
soft ware version 9.1.3. Whenever the ANOVA results showed significant difference
among treatments, mean separation was further performed using least significant
difference (LSD) at 1% or 5% significance level.
3. Results and Discussion
3.1. Physiological status of rose flowers as affected by pulsing preservatives
Water uptake
It is known that cut flowers continue to loss water through transpiration,
which leads to wilting. However, when cut flowers are able to absorb water,
their water balance can better maintained and thus their freshness and vase
life will last longer (Reddy and Singh, 1996). Although there was slight water
balance reduction, flowers pulsed with Aluminum sulfate (T2) was better maintained
followed those pulsed with T3 compared to other pulsing preservatives as indicated
in Figure 1.
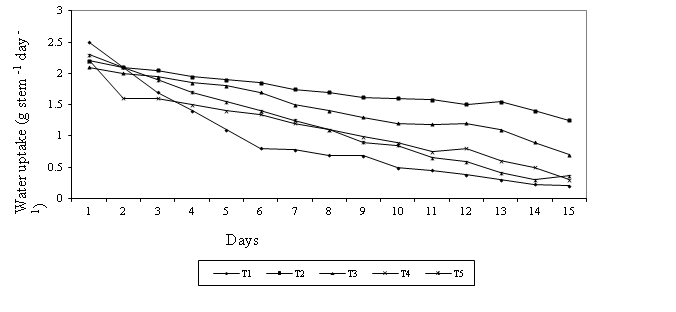
Figure 1. Effect of different pulsing preservatives on
water uptake of rose flowers var. Marcuja during the vase life
T1 = distilled water; T2 = 250 ppm Al2(SO4)3;
T3 = Al2(SO4)3 (250ppm) + Ca(ClO)2
(66.7ppm); T4 = Al2(SO4)3 (666.7ppm) + Ca(ClO)2
(66.7ppm); T5 = Sucrose (20 g l-1)
Water loss through transpiration
According to Halevy (1976) wilting which caused by loss of water is the most
common reason for termination of vase life of cut flowers. Moreover, wilting
is occurred when water loss through transpiration exceeds the rate of water
uptake through cut flowers. As indicated in Figure 2. Aluminum sulfate sustained
stable transpiration rate of cut flowers compared to other pulsing preservatives.

Figure 2. Effect of different pulsing preservatives on
transpiration rate of rose flowers var. Marcuja during the vase life
T1 = distilled water; T2 = 250 ppm Al2(SO4)3;
T3 = Al2(SO4)3 (250ppm) + Ca(ClO)2
(66.7ppm); T4 = Al2(SO4)3 (666.7ppm) + Ca(ClO)2
(66.7ppm); T5 = Sucrose (20 g l-1)
Water balance
In this study, water balance of the cut flowers was determined by the difference
between water uptake and water loss as indicated by Halevy and Mayak (1981).
According to the author He et al. (2006) wilting is considered as termination
of vase life in many flowers which is mostly caused due to water stress than
natural senescence. On the other hand, the quality and longevity of cut flowers
including roses is determined by water balance, which is influenced by uptake
and respiration of cut flowers (Da Silva, 2003). Accordingly, wilting of cut
flowers is commonly occurred when the loss of water of cut flowers through transpiration
is greater than the volume of water taken by cut flowers (Halevy and Mayak,
1981). The results in Figure 3 indicated that the water balance of rose flowers,
which were pulsed by aluminum sulfate (T2), showed the least negative water
balance followed by T3, which confirmed relatively balanced rates of uptake
and respiration of water during their vase life.

Figure 3. Effect of different pulsing preservatives on water balance of rose
flowers var. Marcuja during the vase life
T1 = distilled water; T2 = 250 ppm Al2(SO4)3;
T3 = Al2(SO4)3 (250ppm) + Ca(ClO)2
(66.7ppm); T4 = Al2(SO4)3 (666.7ppm) + Ca(ClO)2
(66.7ppm); T5 = Sucrose (20 g l-1)
Flower weight percentage
Flower weight was assessed daily. Accordingly, the percentage decrease in flower
weight was minimal in aluminum sulfate pulsed rose flowers followed by those
pulsed with treatment T3, which was relatively stable up to 11th day when compared
with other preservatives as well as control treatment (Figure 4).
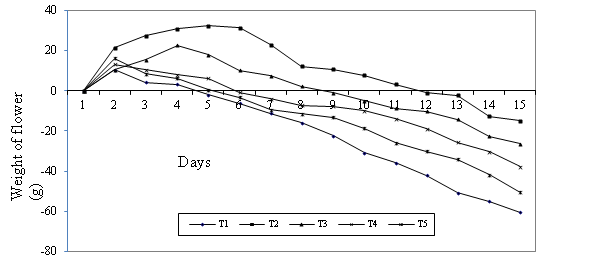
Figure 4. Effect of different pulsing preservatives on percentage of fresh weight
loss during the vase life of rose flowers var. Marcuja
T1 = distilled water; T2 = 250 ppm Al2(SO4)3;
T3 = Al2(SO4)3 (250ppm) + Ca(ClO)2
(66.7ppm); T4 = Al2(SO4)3 (666.7ppm) + Ca(ClO)2
(66.7ppm); T5 = Sucrose (20 g l-1)
Vase life
The vase life of the cut rose flowers was determined by the number of days
in which 50% of the flowers in the flask were bent over. According to the results,
vase life of rose flowers pulsed with aluminum sulfate alone (T2) and treatment
T3 were extended up to 14 and 9 days, respectively (Table 2). The vase life
of control cut roses (T1) as well as those pulsed with treatment T4 and T5 were
generally low compared to those pulsed with other pulsing preservatives. The
extended vase life of those flowers is obviously associated with the stable
water balance in aluminum sulfate pulsed cut flowers. The stable water balance
on the other hand is probably due to the antimicrobial effect of aluminum sulfate,
which reduced the proliferation of bacteria in vase solution responsible for
blockage xylem and thus reduction of vase life (Van Doorn, 1997; De Stigter,
1981; Van Doorn et al., 1990; Liao et al.2000, He et al., 2006). Application
of aluminum sulfate in vase solutions has been also reduced bacterial blockage
of xylem vessel of cut flowers in the findings of various researchers (Liao
et al., 2000; Tsegaw et al., 2011; Seyf et al., 2012; Hussen and Yassin, 2013).
Moreover, buildup of antimicrobial compounds like metal salts from aluminum
sulfate prevent and/or slowdown bacterial growth and ensure proper water uptake
and thus delay senescence and prolong the vase life of cut flowers (Liao et
al. (2000) and Särkkä (2005). Furthermore, aluminum sulfate acidifies
the vase solutions and diminishes bacterial growth (Liao et al., 2000; Hassanpour
et al., 2004) and acts as a bacterial filter by forming Al (OH)3 sediment on
the cut surface of stem (Henriette and Clerkx, 2001). However, use of highly
concentrated aluminum sulfate may reduce the vase life of cut flowers as observed
in treatment T4 of this study, which is also indicated by Lama et al. (2013).
The reduced vase life on sugar pulsed cut flowers in this study may be associated
with the proliferation of bacterial growth in sugar solution which cause plugging
of vascular vessels and reduction of water uptake and thus acceleration of flower
senescence as observed by Ichimura, 2003; Pun and Ichimura, (2003) and Särkkä,
2005. In this regard, Ichimura et al. (2003) obtained reduced vase life of flowers
due to high bacterial growth in sucrose solution. Therefore, they advised the
use of sucrose preservatives in combination of chemicals having antimicrobial
effects.
Table 2. Effect of different pulsing preservatives on percentage
wilted Maracuja variety of rose flowers during vase life (days)
Treatment |
Percentage wilted flowers |
Days |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
| T1 |
|
0 |
0 |
5.6 |
27.8 |
55.6 |
77.8 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
| T2 |
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
16.7 |
27.8 |
33.3 |
38.9 |
44.4 |
61.1 |
| T3 |
|
0 |
0 |
0 |
0 |
5.6 |
11.1 |
22.2 |
27.8 |
33.3 |
50 |
66.7 |
100 |
100 |
100 |
100 |
| T4 |
|
0 |
0 |
0 |
11.1 |
16.7 |
27.8 |
38.9 |
55.6 |
66.7 |
88.9 |
100 |
100 |
100 |
100 |
100 |
| T5 |
|
0 |
0 |
5.6 |
5.6 |
22.2 |
38.9 |
44.4 |
61.1 |
72.2 |
100 |
100 |
100 |
100 |
100 |
100 |
T1 = distilled water; T2 = 250 ppm Al2(SO4)3;
T3 = Al2(SO4)3 (250ppm) + Ca(ClO)2
(66.7ppm); T4 = Al2(SO4)3 (666.7ppm) + Ca(ClO)2
(66.7ppm); T5 = Sucrose (20 g l-1)
3.2. Flower head diameter, fresh weight and vase life of rose flowers as affected
by aluminum sulfate concentrations
In the first set of the experiment aluminum sulfate with the concentration
of 250ppm alone was the best pulsing preservative that maintains the vase life
of rose flowers as long as possible. The purpose of the second set of the experiment
was to determine the optimum aluminum sulfate concentration specific to the
conditions prevailed in the study area, as the concentration of aluminum sulfate
used in the first set was obtained from research results done elsewhere.
Flower head diameter
Rose flowers, which are destined for export market, are mostly harvested as
matured bud with enclosed sepals. The flower head diameter measured at the time
of full bloom was larger in rose flowers pulsed with 250 ppm concentration of
aluminum sulfate compared to other concentrations (Figure 5). The improved flower
head diameter is probably associated with improved water uptake and thus better
water balance of the flowers as indicated in the first experiment of this study.
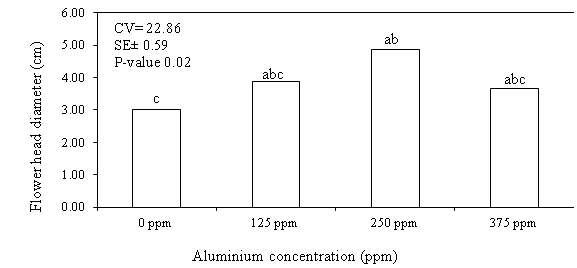
Figure 5. Effect of different rates of aluminum sulfate
on flower head diameter of rose flowers var. Marcuja at full bloom
CV = Coefficient of variation; SE± - Standard error; P-value = probability
value; Means following with the same letter(s) are not statistically different
Generally, the tested aluminum sulfate concentrations have positive effects
on both vase life and fresh weight of Marajuca flowers (Table 3 and Figure 6).
Rose flowers pulsed with 250 ppm concentration aluminum sulfate maintained their
vase life to more than 13 days. The vase life of rose flowers pulsed with 125
ppm and 375 ppm of aluminum sulfate prolonged to more than 10 and 9 days, respectively;
while those pulsed with distilled water terminated their vase life in about
4 days.
Reduction of fresh weight resulted from reduced water uptake is the sign of
vase life termination in cut flowers. The fresh weight of rose flowers pulsed
with 250 ppm of aluminum sulfate was significantly higher than those rose flowers
pulsed with other concentrations as indicated in Figure 6. The results of this
study showed that aluminum sulfate at the concentration of 250 ppm maintained
the freshness and thus prolonged the vase life of Maracuja rose flowers; which
is similar as the results obtained in the first set of the experiment. In agreement
with these findings, Ichimura et al. (2006) and Särkkä (2005) reported
that aluminum sulfate with the concentration of 250 ppm gave the longest vase
life of rose flowers. Similarly, the longest vase life of various varieties
of rose flowers was observed by the application of 250 ppm aluminum sulfate
in the findings of various researchers (Seyf et al., 2012; Hussen and Yassin,
2013; Lama et al., 2013).
Table 3. Effect of aluminum sulfate concentration on percentage
wilted rose flowers var. Maracuja during vase life (days)
Treatment |
Percentage wilted flowers |
Days |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
| 0ppm |
|
0 |
0 |
11.1 |
33.3 |
61.7 |
88.9 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
| 125ppm |
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
5.6 |
16.7 |
27.9 |
55.6 |
66.7 |
94.4 |
100 |
100 |
| 250ppm |
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
22.2 |
38.9 |
55.6 |
77.8 |
| 375ppm |
|
0 |
0 |
0 |
0 |
0 |
0 |
5.6 |
16.7 |
33.3 |
61.1 |
83.3 |
100 |
100 |
100 |
100 |
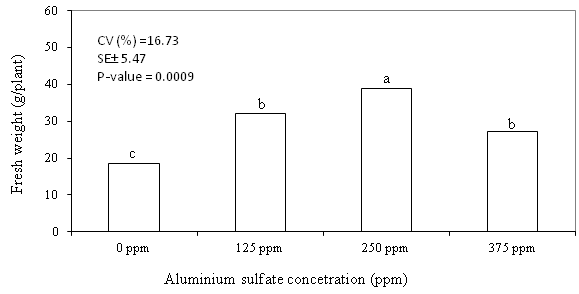
Figure 6. Effects of aluminum sulfate on fresh weight of
rose flowers var. Maracuja during the vase life (14 days after pulsing
CV = Coefficient of variation; SE± - Standard error; P-value = probability
value; Means following with the same letter(s) are not statistically different
4. Conclusion
The results of the present study showed that pulsing with appropriate preservative
solutions maintained the freshness of rose flowers as long as possible and prolonged
their vase life. Rose flowers pulsed with aluminum sulfate at concentration
of 250 ppm prolonged their vase life for more than 13 days and produced bigger
flower heads compared to other concentrations of aluminum sulfate. Moreover,
percent in reduction of fresh weight of those flowers pulsed with 250 ppm of
aluminum sulfate was minimal compared to those pulsed with other preservatives
during vase life which was due to better water uptake and thus stabilized water
balance. Based on the results, it is advised to incorporate 250 ppm of aluminum
sulfate holding solution at production phase to maintain vase life and freshness
of export oriented-rose flowers as well as those for local market.
Acknowledgement
The authors acknowledge Tana Flora PLC and its staffs for their support.
References
|
Butt, S.J. (2003). A review on prolonging the vase life of roses. Pakistan Rose Annual, Published by Pakistan National Rose Society. |
| Chapman, K.D. and Austin-Brown, S. (2007). Methods for extending the freshness of cut flowers, ornamental trees, and plant cuttings. http://www.freepatentsonline.com/7199082.html. (accessed September, 2014). |
| Da Silva, J.A.T. (2003). The cut flower: Postharvest considerations. J. Bot. Sci.3: 406-442. |
| De Stigter, H.C.M. (1981). Effects of glucose with 8-hydroxyquinoline sulfate or aluminum sulfate on the water balance of cut `Sonia' roses. Z. Pflanzenphys. 101: 95-105. |
| EHPEA (2008). Flowers and ornamentals. http://www.ethiomarket.com/eic. (accessed September, 2014). |
| EHPEA and EHDA (2011). Exporting fruit and vegetables from Ethiopia. Ethiopian Horticulture Producers and Exporter Association, Addis Ababa, Ethiopia. |
| Elgimabi, M.E. (2011). Vase life extension of rose cut flower (Rosa hybrida) as influenced by silver nitrate and sucrose pulsing. Am. J. Agri. Biol. Sci.6(1): 128-133. |
| Gerailoo, S. and Ghasemnezhad, M. (2011). Effect of salicylic acid on antioxidant enzyme activity and petal senescence in ‘Yellow Island’ cut rose flowers. J. Fruit Ornam. Plant Res. 19(1): 183-193. |
| Halevy, A.H. and Mayak, S. (1981). Senescence and postharvest physiology of cut flowers- part 2. Hortic. Rev.3: 59-143 |
| Hassanpour, A.M., Hatamzadeh, A. and Nakhai, F. (2004). Study on the effect of temperature and various chemical treatments to increase vase life of cut rose flower “Baccara”. Agric. Sci. Res. J. Guilan Agric. Faculty 1(4): 121-129. |
| Henriette, M.C. and Clerkx, A.C.M. (2001). Anatomy of cut rose xylem observed by scanning electron microscope. Acta Hortic. 547: 329-339. |
| Hussen, S. and Yassin, H. (2013). Review on the impact of different vase solutions on the postharvest life of rose flower. Int. J. Agri. Res. Rev. 1(2): 013-017. |
| Ichimura, K., Kawabata, Y., Kishimoto, M., Goto, R. and Yamada, K. (2003). Shortage of soluble carbohydrates is largely responsible for short vase life of cut ‘Sonia’ rose flowers. J. Jap. Soc. Hortic. Sci.72: 292-298. |
| Ichimura, K., Taguchi, M. and Norikoshi, R. (2006). Extention of the vase life in cut roses by treatment with glucose, isothiazolinonic germicide, citric acid and aluminum sulphate solution. Jap. Agri. Res. Quart. 40(3): 263- 269. |
| Lama, B., Ghosal, M., Gupta, S.K. and Mandal, P. (2013). Assessment of different preservative solutions on vase life of cut roses. J. Ornam. Plants (J. Ornam. Hortic. Plants) 3(3): 171-181. |
| Liao, L.J., Lin, Y.H., Huang, K.L., Chen, W.S. and Cheng, Y.M. (2000). Postharvest life of cut rose flowers as affected by silver thiosulfate and sucrose. Bot. Bull. Acad. Sinica 41: 299 - 303. |
| Loubaud, M. and Van Doorn, G. (2004). Wound-induced and bacteria-induced xylem blockage in roses, Astible and Viburmum. Postharvest Biol. Technol. 32(3): 281-288. |
| Lutz, J.M. and Hardenburg, R.E. (1968). The commercial storage of fruits, vegetables and florist and nursery stock. Department of Agriculture Agricultural Research Sevice , USA. |
| Minstry of Trade and Industry (MoTI) (2014). Export performance evaluative report of 2006/07 Ethiopian fiscal year. Ministry of Trade and Industry, Addis Ababa, Ethiopia. |
| Nowak, J. and Rudnicki, R. (1990). Postharvest Handling and Storage of Cut Flowers, Florist Greens, and Potted Plants. Timber Press, Inc., Portland. |
| ProFlower (2012). How to choose the right rose for every occasion. http://www.proflowers.com/blog/. (accessed February 28, 2016). |
| Pun, U.K. and Ichimura, K. (2003). Role of sugars in senescence and biosynthesis of ethylene in cut flowers. Jap. Agri. Res. 37: 219-224. |
| Särkkä, L. (2005). Yield,quality and vase life of cut roses in year round greenhouse production. PhD Dissertation, University of Helsinki, Finland. |
| Seyf, M., Khalighi, A., Mostofi, Y. and Naderi, R. (2012). Study on the effect of aluminum sulfate treatment on postharvest life of the cut rose ‘Boeing’ (Rosa hybrid cv. Boeing). J. Hortic. Forest. Biotech. 16(3): 128-132. |
| Subhashini, R.M.B., Amarathunga1, N.L.K., Krishnarajah, S.A. and Eeswara, J.P. (2011). Effect of Benzylaminopurine, Gibberellic Acid, Silver Nitrate and Silver Thiosulphate, on postharvest longevity of cut leaves of Dracaena. Ceylon J. Sci.(Bio. Sci.) 40(2): 157-162. |
| Synge, P.M. (1971). The dictionary of rose in color. 1st Edn., Madison Square Press, New York, USA. |
| Tsegaw, T., Tilahun, S. and Humphries, G. (2011). Influence of pulsing biocides and preservative solution treatment on the vase life of cut rose (Rosa hybrid L.) varieties. J. Appl. Sci. Techn. 2(2): 1-18. |
| Van der Maden, E., Hoogerwerf, F., Marrewijk, V., Kerklaan, E., Posthumus, J., van Boven, A., Elings, A., Victoria, N.G., Rikken, M. and Humphries, G., Eds. (2011). Handbook for greenhouse rose production Ethiopia. DLV Plant, Wageningen, The Netherlands |
| Van Doorn, W.G., De-Witte, Y. (1997). Sources of the bacteria involved in vascular occlusion of cut rose flowers. J. Am. Soc. Hortic. Sci 122: 263-266. |
| Van Doorn, W.G., De-Witte, Y. and Perik, R.R.J., (1990). Effect of antimicrobial compounds on the number of bacteria in stems of cut rose flowers. J. Appl. Bacteriol. 68: 117-122. |
| Zlesak, D.C. (2006). Issues, challenges and opportunities for the 21th Century. Rosa x hybrida. In: Flower breeding and genetics, pp. 695-738 (Anderson, N. O. eds.). Springer, Netherlands. |